How does lunar dust turn into oxygen?
Technology reporter
 Plot gap
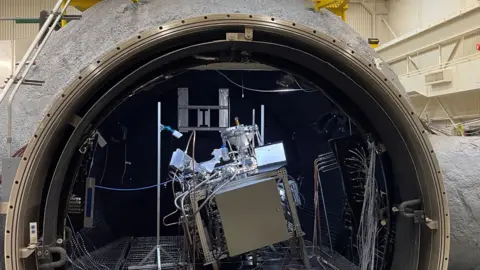
Plot gapInside a giant sphere, the engineers huddled over their tools. Before them stood a silver metal shield encased in colorful wires—a box they hoped would one day produce oxygen on the moon.
Once the team released the sphere, the experiment began. The box-like machine was now ingesting a small amount of dusty regolith – a mixture of dust and sharp particles with a chemical composition that mimicked real lunar soil.
Soon that regolith darkened. A layer is heated to a temperature above 1,650C. And when some reactants were added, oxygen-containing molecules began to bubble up.
“We’ve tried everything we can on Earth right now,” said Brant White, program manager at private company Sierra Space. “The next step is to go to the moon.”
The Sierra Space Experiment opened this summer at NASA’s Johnson Space Center. Researchers are far from the only technologies that are developing systems that will provide astronauts living on the base of the moon in the future.
Those astronauts need oxygen to breathe, but they also make rocket fuel for spacecraft that take off from the moon and go to other places – including Mars.
Moonbase dwellers can also search for iron and collect it from the dusty gray debris that litters the lunar surface.
Much depends on building reactors that can efficiently extract such resources.
“It could save billions of dollars in mission costs,” Mr. White said.
 Plot gap
Plot gapFortunately, the lunar regolith is full of iron oxides. But while the science of extracting oxygen from iron oxides, for example, is well understood on Earth, doing so on the Moon is very difficult. Not because of circumstances.
The giant spherical chamber, which hosted the Sierra Space Experiments in July and August this year, created a vacuum and simulated the moon’s temperatures and pressures.
The company says it has had to make improvements to how the machine works over time so that it can better cope with the extremely brittle and corrosive texture of the regolith. “It gets everywhere, it exhausts all kinds of methods,” Mr. White said.
And one thing, crucially, that you can’t test on Earth or even when orbiting our planet is the Moon’s gravity – which is one-sixth that of Earth. It may not be until 2028 or later that Sierra Space will test the system on the Moon using real regolith in low gravity.
 NASA
NASAUnless engineers design it, the moon’s gravity could be a problem for some oxygen-producing technologies, said Paul Burke of Johns Hopkins University.
In April, he and his colleagues He published a paper It details the results of computer simulations that show how the moon’s relatively weak gravity would hamper the process of extracting specific oxygen. The process being investigated here was electrolysis of molten regolyse, using electricity to break down oxygen-containing lunar minerals to extract oxygen directly.
The problem is that such technology works by creating oxygen bubbles on electrodes in the molten regolith itself. “It is the consistency of honey. It’s very, very viscous,” said Dr. Burke.
“Those bubbles don’t rise as quickly—and they can be delayed in detaching from the electrodes.”
There may be ways around this. One could be shaking the oxygenator, which shakes the bubbles freely.
And overly soft electrodes may cause oxygen bubbles to separate easily. Dr. Burke and his colleagues are now working on such ideas.
Sierra Space Technology, the carbonization process, is different. In their case, when oxygen-containing bubbles are formed in the regolyze, they do so freely, not from the surface of the electrode. Mr White says they are less likely to stick.
Highlighting the value of oxygen for future lunar missions, Dr. Burke estimates that an astronaut would need about two or three kilograms of regolith per day, depending on the astronaut’s fitness and activity level.
However, the lunar base’s life support systems can recycle the oxygen breathed by the astronauts. In that case, it wouldn’t be necessary to make a lot of regolith just to keep lunar inhabitants alive.
Dr. Burke added that the real use case for oxygen extraction technologies is in providing oxidizers for rocket fuels, which will enable great space exploration.
 MIT and Shaan Jagani
MIT and Shaan JaganiObviously, the more resources that can be made on the moon, the better.
Sierra’s space system requires some carbon addition, although the company says it can recycle most of this after each oxygen-generating cycle.
Palak Patel, a doctoral student at the Massachusetts Institute of Technology, came up with an experiment with his colleagues. Molten regolith electrolysis systemTo extract oxygen and iron from lunar soil.
“We’re really looking at it from the perspective of, ‘Let’s try to reduce the number of resupply missions,'” she said.
In designing their system, Ms. Patel and her colleagues identified a problem identified by Dr. Burke: Low gravity can hinder the separation of oxygen bubbles that form on the electrodes. To counter this, they used a “sonicator”, which blasts the bubbles with sound waves.
Ms Patel says that in the future, mining machines on the moon will be able to extract, for example, iron, titanium or lithium from the regolith. These materials could help lunar astronauts make 3D-printed parts for their lunar base or replacement parts for damaged spacecraft.
The significance of the lunar regality does not end there. Ms. Patel said that, in separate experiments, she melted simulated regolith into a solid, dark, glassy substance.
She and her colleagues worked out how to turn this material into solid, hollow bricks that could be useful for building structures on the moon. A compelling black monolithTell them. why not?


